Documents
Component Overview
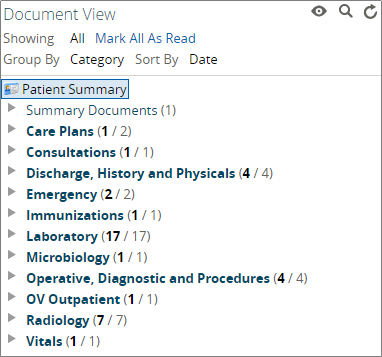
To supplement the information located in the "Patient Summary" view, each patient's record also includes a "Document View" component. This component, located along the left-hand side of the "Patient Summary" view, includes an expansive array of notes, reports, and results for the selected patient.
Examples of the types of documents contained in the Clinical Portal include:
Care Plans
Consultations
Discharge, History, and Physical Notes
Hospital, ED, and PCP Visit Notes
Immunization and Vitals Information
Radiology, Microbiology, and Laboratory Results
Summary Documents
Operative, Diagnostic, and Procedural Notes
CCDAs (for more information, visit the section on “CCDA Information”)
Key Functionality
Content Organization
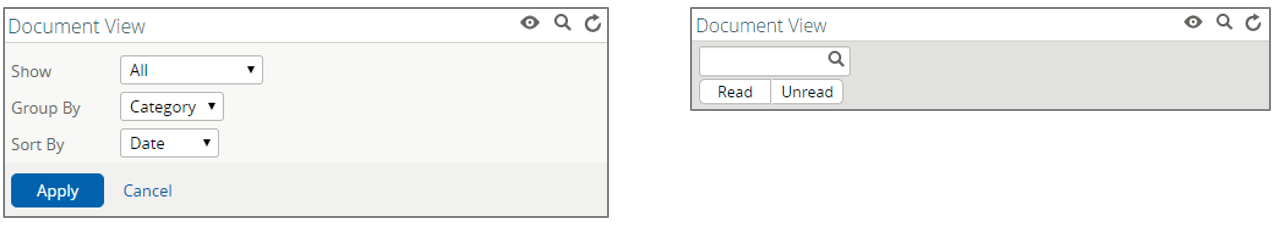
Contents contained in the "Document View" will vary by patient based on their specific encounters and/or data received from participating organizations. By selecting the eye icon, users can configure the display of contents in the component according to their preferences:
Function | Description |
|---|---|
Show | Options to show all documents or only those received within a certain period of time |
Group By | Options to group the documents by Category, Date, Service, or Provider |
Sort By | Options to sort the ordering of documents by Date, Title, or Provider |
Alternatively, users may choose to select the magnifying glass icon to search the component by keyword.
Abnormality Color Coding
Within certain document categories, additional features and functionality exist. For example, in the "Laboratory" document category, color-coding (derived from the resulting laboratory) is used to quickly call attention to results of interest, including:
Color | Description | Example |
|---|---|---|
Black | Normal/expected range |  |
Yellow | Abnormal range | |
Red | Critical range |
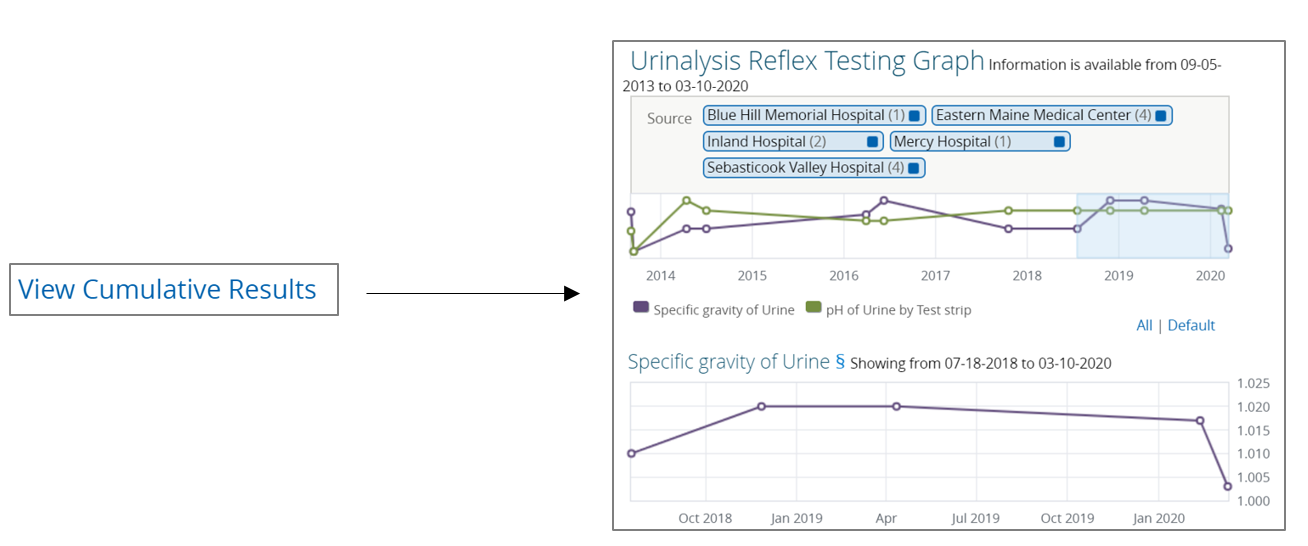
Cumulative Results Trending
Upon selecting a particular document, users can often graph the patient's results over time by selecting the "View Cumulative Results" hyperlink located to the right of the document title.